Coppel Laboratory

Ross Coppel
Position: Professor of Microbiology and Director of the Victorian Bioinformatics Consortium

MY CONTACT
Address: Department of Microbiology, Monash University, Wellington Road, Clayton, Victoria, 3800, Australia
Email: ross.coppel@ med.monash.edu.au

Research Interests
Malaria:
Mycobacterial Infections:
Bioinformatics:
Autoimmune Liver Disease:

LINKS
Malaria Protein Structure and Function

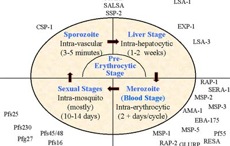
Human malaria caused by the protozoan Plasmodium falciparum is a major public health problem of many countries in tropical areas. Hundreds of millions of people are infected with malaria every year and this results in several million deaths annually, predominantly in children. The increasing frequency of drug resistant parasites and the breakdown of malaria control measures in many countries has further worsened the situation. Research progress in the laboratory focuses on understanding facets of the basic biology of the parasite and its relationship to the human host, together with studies to identify components of the parasite, that may be used in vaccines. Research projects within the laboratory make use of a wide range of techniques: from cell biology, such as in vitro culture of the parasite, metabolic labelling studies, immunochemistry to those of state-of-the-art molecular biology.
Interactions between malaria proteins and the human red blood cell
During malaria infection, the parasite lives inside the human red blood cell and remodels this cell extensively, changing both its structural and functional properties. The process by which the red blood cell is modified involves the insertion of new parasite proteins into the membrane and membrane skeleton of the red blood cell. We are interested in defining at the molecular level which parasite proteins are involved, and identifying the precise regions of both parasite and host proteins that bind to each other. In this way we hope to understand how parasitization affects red blood cell structure and interferes with normal red cell function.
Interactions between the malaria infected cell and the vascular wall
When red blood cells become infected with mature forms of P. falciparum, they adhere to the walls of blood vessels. This adherence phenomenon, especially if it occurs in blood vessels of the brain, is part of a complicated process that ultimately leads to severe malaria. We are undertaking studies at the cell that quantify this adhesion level and to understand the factors that enable parasites to adhere to the vascular endothelial cells and specific receptor molecules. In these studies, parasites are introduced into flow chambers and examined by direct microscopy to determine their adhesive behaviour under conditions of physiologically relevant flow. Several factors such as the role of parasite accessory proteins and antigenic variation of the adherence ligand are currently being examined.
Molecular biology of malaria antigens
P. falciparum is a complex organism that synthesizes thousands of proteins during its life cycle. The recent availability of a full genome sequence of the malaria parasite provides information about every protein encoded by the parasite. The function of many of these proteins is unknown and some may be proteins involved in many important parasite functions including invasion of the red blood cell. We are interested in cloning the genes of proteins involved in invasion and understanding the complex interaction of these proteins. With this information it may be possible to design new drugs that interfere with the invasion process and hence malarial disease. Alternatively, such proteins may be useful components of a vaccine against malaria.
The Malaria Vaccine Group
Prof. Coppel oversees a project funded by the Malaria Vaccine Initiative to develop two promising antigens for use in human vaccine trials. A process to produce the antigens (merozoite surface proteins 4 and 5) at an industrial scale under good manufacturing practice standards is being developed in collaboration with an industry partner, CSL Ltd. The current project staff are Svetozar Kovacevic, Harini de Silva, Khosse Mitri and Valentina Dubljevic. Registered users can access further information here.
Seroepidemiology of malaria infection
The protozoan parasite Plasmodium falciparum induces a complex immune response in the human host against hundreds of different parasite proteins. The kinetics of acquisition of the antibody response and the relationship of particular antibody specificities to the immune state are still poorly described. The recent availability of recombinant expressed proteins of Plasmodium falciparum allows such experiments to be performed with a precision never before possible. Sera taken from patients with defined immune status will be examined for the presence of antibodies to a number of different malaria antigens. In this way we hope to identify parasite proteins that may be useful components of a malaria vaccine.
Production and delivery of malaria vaccines using novel delivery methods including transgenic plants
Edible Malaria Vaccine Group
The success of immunisation strategy against malaria will depend principally on vaccine production, distribution and delivery. Malaria is almost exclusively a disease in developing and often economically disadvantaged countries, where the cost of a vaccine is a significant impediment to widespread deployment. Therefore a technology that enables low cost of production of antigens capable of achieving protective efficacy and ease of administration would be a major advance. We are looking at alternate approaches to vaccination including DNA vaccines, new adjuvants for protein immunogens, novel viral vectors such as adeno-associated virus and transgenic plants that express important parasite antigens. These methods alone or in combination may be more effective than existing approaches.
We are developing transgenic lettuce plants that can be used as edible vaccines. Such plants could be grown locally, reducing costs and transport requirement. Oral administration of these vaccines offers a cheap, practical and non-invasive means of inoculation that can easily be given in multiple doses. To date we have shown that a host-protective immune response can be generated in mice that have been orally immunised with recombinant malaria proteins. We have also shown that these proteins can be expressed in plants. We are now working on strategies to improve the expression level of antigens in plants and investigate the feasibility of using plants as edible malaria vaccines. If successful, this approach will make a promising strategy for providing affordable vaccines for the less developed world.